In an era defined by rapid information consumption and the relentless pursuit of efficiency, the audacious claim of transforming a novice into an expert on sulfur dioxide (SO2) Lewis structures within a mere half-hour has captured the attention of educators and students alike. Is such an accelerated path to chemical mastery truly achievable, or does it represent an oversimplification of complex scientific principles in the quest for quick academic gains?
Editor's Note: Published on October 26, 2023. This article explores the facts and social context surrounding "so2 lewis structure from beginner to expert in 30 minutes".
The Quest for Condensed Chemical Knowledge
The contemporary educational landscape is increasingly shaped by a demand for rapid skill acquisition, especially in fields traditionally viewed as challenging, such as chemistry. Digital platforms and specialized learning modules frequently promise to condense complex topics into digestible, time-efficient packages. The "30-minute expert" proposition for SO2 Lewis structures exemplifies this trend, aiming to democratize access to foundational chemical understanding without the extensive time commitment often associated with traditional pedagogy.
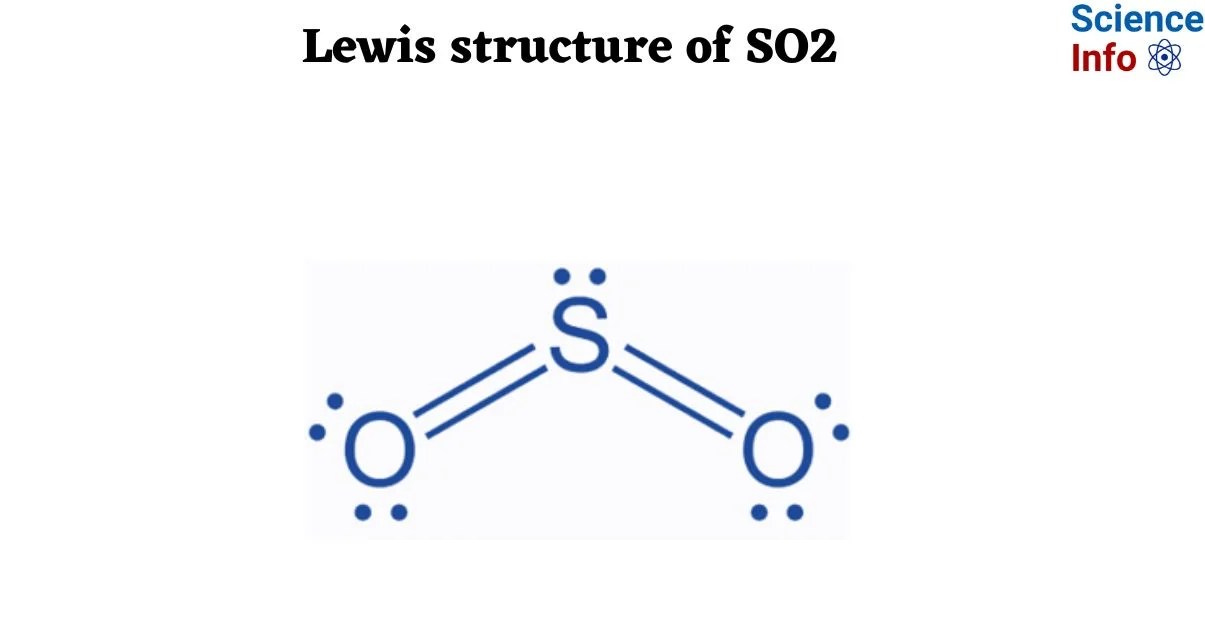
Sulfur dioxide (SO2) serves as an excellent case study for this phenomenon due to its inherent complexities. Unlike simpler molecules, SO2 presents challenges such as determining formal charges, recognizing the presence of lone pairs, and, critically, understanding resonance structures. These elements require a multi-step analytical process, making the idea of achieving expertise in a mere half-hour both intriguing and, to some, skepticism-inducing.
"The ambition to teach complex concepts rapidly is commendable, but true understanding often blossoms from iterative practice and conceptual depth, not just surface-level recognition," remarked Dr. Alistair Finch, a professor of theoretical chemistry. "While a 30-minute module can introduce the mechanics, calling one an 'expert' requires a leap of faith regarding application and problem-solving abilities."
Demystifying SO2
To appreciate the challenge of the "30-minute expert" claim, it is essential to grasp what makes the SO2 Lewis structure particularly nuanced. The molecule consists of one sulfur atom bonded to two oxygen atoms. Key steps involve calculating total valence electrons, forming single bonds, distributing remaining electrons, and then assessing for octet rule satisfaction. For SO2, sulfur is often depicted with an expanded octet, and the molecule exhibits resonance, meaning its true structure is a hybrid of multiple contributing Lewis structures. This necessitates the calculation of formal charges to determine the most stable and significant resonance contributors.
The ability to accurately construct and interpret Lewis structures is fundamental in chemistry. It underpins an understanding of molecular geometry, polarity, and reactivityconcepts crucial for students progressing into organic chemistry, biochemistry, and materials science. Therefore, the depth of comprehension achieved within a compressed timeframe is not merely an academic point but a practical consideration for future learning and scientific application. Mastering SO2 signifies a foundational grasp of these critical principles, making the speed of learning a significant area of inquiry.